
Ideal if youre a student, teacher or just have an interest in the chemical sciences. Click on 'Element Atomic Number', 'Element Symbol', 'Element Name' and 'Element Atomic Radius (Calculated)' headers to sort. This fact-filled, image-rich app is the only periodic table you need. This fact has key implications for the building up of the periodic table of elements.This Atomic Radius (Calculated) table gives the Atomic Radius (Calculated) of all the elements of periodic table in pm. Contained in the nucleus are the protons and neutrons. Each element has a symbol, which is one or two letters. The periodic table lists the elements in order of increasing atomic number. Each element is identified by the number of protons in its atoms. Elements that have similar chemical properties are. The ordering of the electrons in the ground state of multielectron atoms, starts with the lowest energy state (ground state) and moves progressively from there up the energy scale until each of the atom’s electrons has been assigned a unique set of quantum numbers. Atomic Structure and the Periodic Table Foundation and Higher. There are 118 elements on the periodic table. A modern periodic table lists elements left to right by atomic number. More Filters Nonmetals States Periods 1 H Hydrogen 1.008 2 He Helium 4.003 3 Li Lithium 6.941 4 Be Berylium 9. Each one has a different number of protons, electrons and neutrons. Atomic Number of Elements There are about ninety elements found on Earth. He organized the elements by atomic number, which is equal to the number of protons found in the nucleus of the element’s atoms. The table below consists of 118 elements of the periodic table, sorted by atomic number, atomic weight, symbols, density, discovered year and the group. It is the Pauli exclusion principle that requires the electrons in an atom to occupy different energy levels instead of them all condensing in the ground state. The periodic table of the elements was first introduced in the mid-19th century by Dmitry Mendeleev. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the atom. The modern periodic table is based on Dmitri Mendeleevs 1896 observations that chemical elements can be grouped according to chemical properties they. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10 -19 coulombs. The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. There is also a 2019 edition of this table.
#Atomic table pdf
Here is the pdf file of the color periodic table so you can save and print it. It includes element names, symbols, atomic numbers, atomic weights, element groups, and periods.
#Atomic table free
The total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol Z. 2013 Edition This free periodic table wallpaper has a white background. In the periodic table, the elements are listed in order of increasing atomic number Z. Periodic Table of the Elements, With Symbols - Science Quiz: Memorizing the names of all the elements can be tough. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. The configuration of these electrons follows from the principles of quantum mechanics. Since 1899 the IUPAC Commission on Isotopic Abundances and Atomic Weights ( CIAAW) has been evaluating atomic weights and abundances. The truth is that atomic weights have changed as a function of time. The chemical properties of the atom are determined by the number of protons, in fact, by number and arrangement of electrons. Atomic weights found within a periodic table one might think are constant.
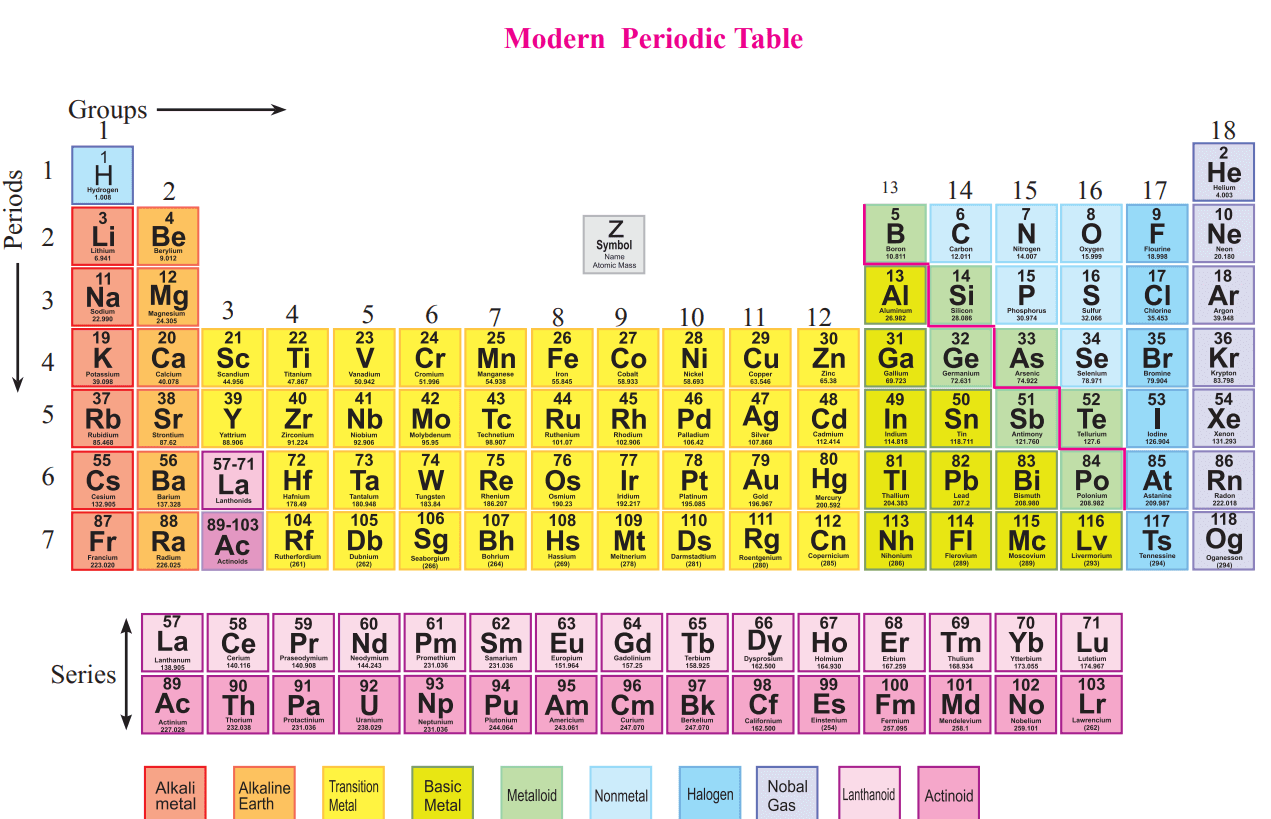
Many of the element symbols are derived from the elemental name.

There is a recurring pattern called the “periodic law” in their properties, in which elements in the same column (group) have similar properties. Generally, within one row (period) the elements are metals to the left, and non-metals to the right, with the elements having similar chemical behaviours placed in the same column.Įvery solid, liquid, gas, and plasma is composed of neutral or ionized atoms. The naming and symbol of the elements in the periodic table is an interesting story itself. It is organized in order of increasing atomic number. In addition to the information contained within the Periodic Table of Elements, the following articles may be helpful if you are writing a report about an. The periodic table is a tabular arrangement of the chemical elements.


 0 kommentar(er)
0 kommentar(er)
